Module 4
EVALUATION &
FOLLOW-UP
At the end of this module, you should be able to:
- Identify Euclid lens parameters from the package labeling
- Describe the process of evaluating a Euclid lens at initial dispense
- Outline the recommended follow-up schedule
Receiving Your Euclid Lenses (1/6)
Your Euclid lenses will arrive in clearly marked packaging that details the individual parameters of the lenses. Each Euclid lens is laser marked with a unique laser ID to enable easy identification of your lens. We will review the package labeling next.
Packaging Labeling(2/6)
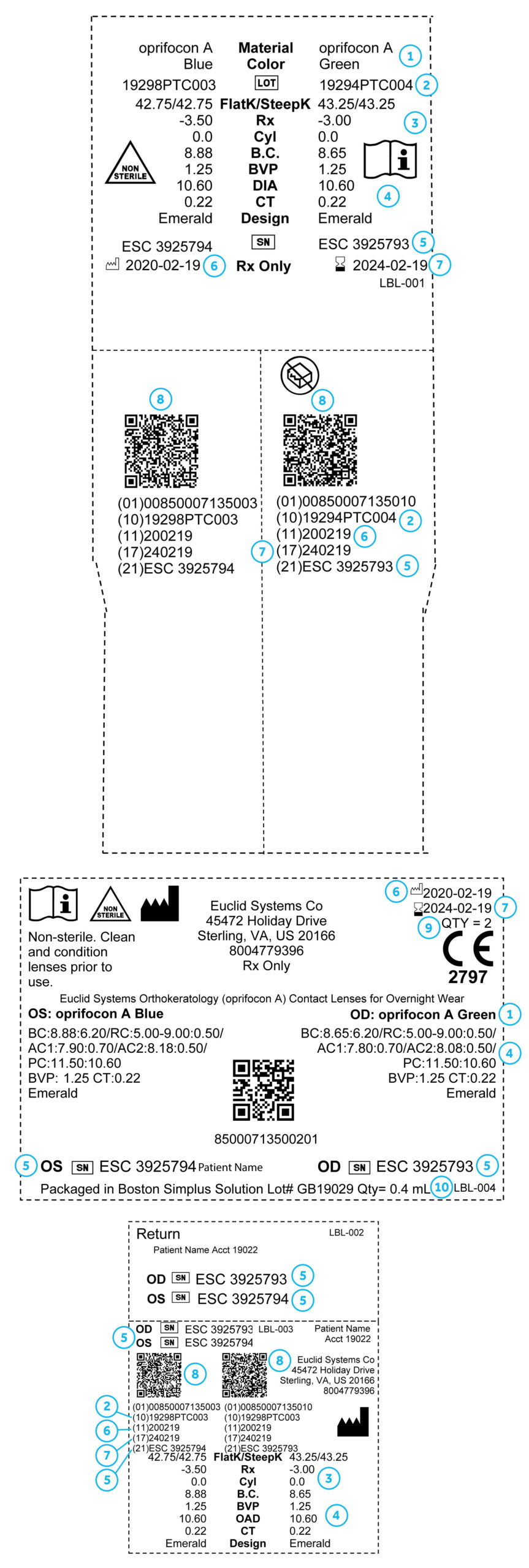
- Manufacturing date
- Expiration date
- Quantity of lenses contained in the package
- Lens material and color
- Each lens parameter – BC, RC, AC1, AC2, PC, BVP, and CT – is noted next to its chord diameter
- Lenses may be shipped in solution; the solution lot # is recorded on your labeling
- Each lens has a unique lens identifier laser marked on the surface
- Device identifier: UDI barcode
- Material lot number
- The patient’s K readings and target RX
Initial Dispense (3/6)
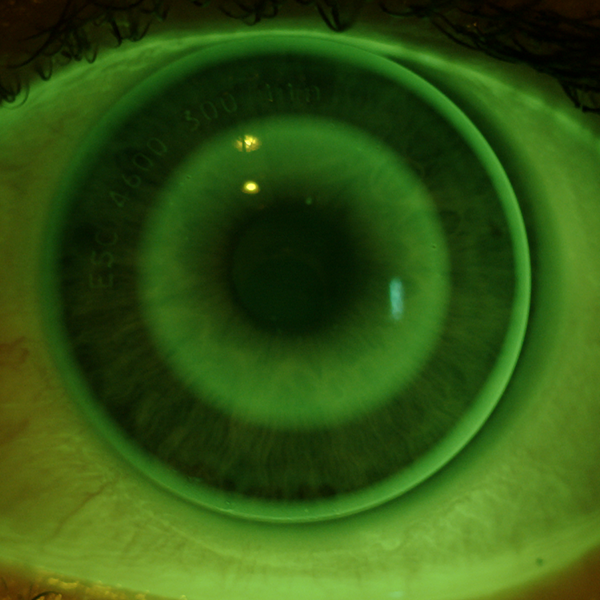
Be sure to clean, disinfect, and condition the lenses prior to use with a solution approved for use with GP lenses. Upon initial dispense, perform a slit lamp evaluation (with and without fluorescein) and look for the following signs of a well-fitting lens:
- General lens centration
- Lens movement with the blink
- Formation of an early “bulls-eye” pattern
Do not dispense the lenses if you see excessive lens movement or no lens movement at all, or if the patient reports immediate pain with the lenses. Remove, re-clean, and reinsert lenses to see if this resolves the issue. Instruct the patient on proper lens insertion and removal, care and cleaning with solution, and the instillation of lubricating drops/artificial tears.
Schedule an initial follow-up for the following morning, as early as possible after the removal of lenses.
First Follow-up Appointment (4/6)
If the patient is wearing the lenses:
- Perform a slit lamp evaluation for lens movement
- Check unaided visual acuity
- Perform a BCVA evaluation
- Check over-refraction
With the lenses removed, perform the following:
- BCVA evaluation
- Sphero-cylinder refraction
- Corneal Topography
- Corneal health check
At the one-night mark, it is possible the patient will not experience full correction and centration may not yet be ideal. Check back in at the one-week mark to ensure that treatment is progressing.
Recommended Follow-up Schedule (5/6)
We recommend that practitioners follow-up with their patients wearing Euclid Ortho-K lenses at regular intervals to ensure that the treatment is progressing as expected. Most patients experience full correction between 14-30 days after starting treatment. As the patient continues to wear the lenses, exams later in the day can be helpful to assess if there are issues with nighttime regression.
Sample follow-up schedule:
- One-week morning visit
- One-month late day visit
- Six-month late day visit
- One-year visit
Summary (6/6)
In this module, we discussed:
- Euclid Ortho-K lenses will arrive with detailed package labeling, indicating your lens parameters
- Upon initial dispense, check for lens movement, and instruct the patient on I&R and lens cleaning and disinfection
- The recommended process for initial dispense and follow-up exams
- The recommended follow-up schedule for patients wearing Euclid lenses